https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3043740/
https://healthjade.com/ivermectin/
Healthcare related, here are some doc interviews I have found interesting: https://youtu.be/k9GYTc53r2oThat is an amazing video. Long but if you have doubts that this crisis is real, you need to watch this. If you are interested in the treatment side of this, it will scare the sh!t out of you what his guest tells about her time at her hospital. She was blunt at one point, when she said, if PPE disappears, she quits. And no she doesn't think that she should be expected to kill herself for a predatory sickcare system that don't give a sh!t about her or other HCW. ADDED: The doctor interviewed says NO to Ibuprofen or Aleve.
Not promoting it. Just looking up the info, and sharing
https://steemkr.com/health/@pomeline/ivermectin-a-possible-treatment-of-coronaviruses-and-ncov
https://www.npr.org/2020/04/02/825200206/doctors-say-hospitals-are-stopping-them-from-wearing-masks
"When she got an assignment last week at Saint Alphonsus Regional Rehabilitation Hospital in Boise, Idaho, she packed her own personal protective equipment and drove to town. She disinfected her hotel room and stayed away from other guests, but worried about the coughing person in the room next door. So she donned her own fitted N95 mask that she uses for work. "I wanted to protect myself," she said. "I wanted to protect my patients." That first day at work, Buckalew said, she was told to take off her mask. When she asked hospital administrators why, the reasons kept changing. First, Buckalew said she was told it was against hospital policy for health care workers to bring their own gear. Then, she said, administrators told her if she wore her own N95 mask, others would want to wear the masks as well and the hospital didn't have enough. Finally, Buckalew said, it was that CDC guidelines don't require the mask at all times. "I said if I can't wear it, then we have a problem," she said. Refusing to take off her mask, she said, got her terminated. Then, she said, after complaining she was reinstated and then terminated again — all within three days."I see lots and lots of law suits coming.
Per the latest video, here is the article about the blue shop towel material for mask filters.
https://www.businessinsider.com/homemade-mask-using-hydro-knit-shop-towel-filters-better-2020-4
Dr Gordon rocks. she is smart and she is genuine and straight shooter. You wont get more real than that.
What are your sources of information that substantiate your claim that Jon Rappaport “has a long history of promoting hoaxes”. I find him to very credible. He is a journalist who actually does the homework before he writes an article.
https://www.youtube.com/watch?v=PnMNBTc1DQU&list=PLQVnQN4_pN5nOO_5s4XXYwIA5p3ZAJJw0
Yeah, a happy enchilada and you think you’re gonna drown…
We’ve been noticing that most patients that go on ventilators do worse and don’t make it. Maybe we are making the wrong assumptions about putting people on ventilators in the first place.
https://www.statnews.com/2020/04/08/doctors-say-ventilators-overused-for-covid-19/
Because U.S. data on treating Covid-19 patients are nearly nonexistent, health care workers are flying blind when it comes to caring for such confounding patients. But anecdotally, Weingart said, “we’ve had a number of people who improved and got off CPAP or high flow [nasal cannulas] who would have been tubed 100 out of 100 times in the past.” What he calls “this knee-jerk response” of putting people on ventilators if their blood oxygen levels remain low with noninvasive devices “is really bad. … I think these patients do much, much worse on the ventilator.” That could be because the ones who get intubated are the sickest, he said, “but that has not been my experience: It makes things worse as a direct result of the intubation.” High levels of force and oxygen levels, both in quest of restoring oxygen saturation levels to normal, can injure the lungs. “I would do everything in my power to avoid intubating patients,” Weingart said. One reason Covid-19 patients can have near-hypoxic levels of blood oxygen without the usual gasping and other signs of impairment is that their blood levels of carbon dioxide, which diffuses into air in the lungs and is then exhaled, remain low. That suggests the lungs are still accomplishing the critical job of removing carbon dioxide even if they’re struggling to absorb oxygen. That, too, is reminiscent of altitude sickness more than pneumonia.Maybe the low O2 level we have been taking as critical data is wrong taking into account the new data on iron and blood cells?
Found a nice summary article in a journal aimed at ophthalmologists. A couple of you have asked for a summary of the evidence to give to a primary doctor. This would be a good link for this purpose.
https://crstoday.com/articles/not-rated/hydroxychloroquine-for-the-treatment-and-prevention-of-covid-19/?fbclid=IwAR2PailCyUfADoyC_OHOYnGr2Conoqt5wIKXIRDpOuBE2ivgWduJXjU-TDo
Hydroxychloroquine for the Treatment and Prevention of COVID-19
A review of the evidence and an explanation of why this drug is a crucial element of prophylaxis for health care workers.
William B. Trattler, MD; Gary Wörtz, MD; and Sean Ianchulev, MD, MPH
Social distancing. Physical distancing. Governmental orders to stay at home. One of the greatest pandemics in the history of the modern world—coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome (SARS) coronavirus (SARS-CoV-2)—has essentially shut down the world’s economy. The reasons underlying these drastic responses are that COVID-19 is easily transmissible; infected individuals are often asymptomatic for a week or more; a small but real percentage of infected patients will end up in hospital intensive care units (ICUs), overwhelming the health care system; and there is a high mortality rate in hospitalized patients because of a cytokine storm that can lead to multisystem organ failure.
There are currently no proven therapies to treat or prevent this novel viral infection. Once contracted, patients—regardless of age or health status—are at risk of hospitalization and even death from COVID-19.
World leaders have advised their citizens to avoid travel, stay at home, and prevent unnecessary contact with others. These measures are especially important for the elderly, the immunocompromised, and infants.1
ON THE FRONT LINES
Health care workers on the front lines of the COVID-19 outbreak continue to care for patients. These providers are often in harm’s way because of a severe shortage of personal protective equipment (PPE). In a recent survey of more than 1,000 hospital and health care representatives, almost half said they were completely or almost out of respirator masks and face shields. Even the supply of hand sanitizer, gowns, and gloves is reaching a critical low.2
Health care workers, because they are in close proximity to patients, are at a high risk of developing COVID-19. A study conducted in Wuhan, China, found that 29% of patients admitted to the hospital from January 1 to January 28 were health care providers.3
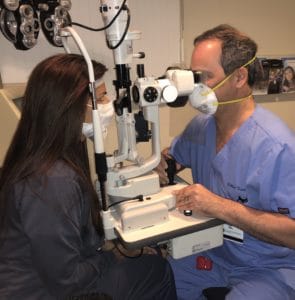
All nonessential health care has been put on hold during this crisis, and ophthalmologists and optometrists are only providing urgent and emergent care. Unfortunately, it is impossible to know for sure whether or not a patient being seen for an eye emergency has COVID-19. Every patient encounter increases the provider’s risk of exposure. The requirement for eye care providers to be physically close to patients to perform an eye examination (Figure 1) has led to a high number of eye care providers to test positive for COVID-19.4
Figure 1. Ophthalmologists and optometrists must sit in close proximity to patients when performing eye exams. Even with face masks and a clear protective shield attached to the oculars of the slit lamp, the close proximity increases the chance of exposure to COVID-19.
HYDROXYCHLOROQUINE FOR PROPHYLAXIS
In a positive development, basic science studies and clinical research support the use of hydroxychloroquine for both prophylaxis and therapeutic treatment of COVID-19. Hydroxychloroquine is a widely available generic medication that is approved by the FDA. The drug has a good safety profile and a very long half-life, and it is commonly used for a variety of indications, including the treatment of lupus and rheumatoid arthritis and the prevention and treatment of malaria.
Basic science studies. Initial research focused on chloroquine, a similar drug to hydroxychloroquine. Performed after the SARS epidemic caused by coronavirus more than 15 years ago,5 the research showed that chloroquine demonstrated antiviral activity against SARS-CoV in vitro. Hydroxychloroquine, which has a better safety profile than chloroquine, has also demonstrated in vitro efficacy against SARS-CoV.6
Earlier this year, after the outbreak of COVID-19 in China, clinicians there noticed that none of the first 178 COVID-19 patients admitted to the hospital had lupus.7 The clinicians then evaluated 80 lupus patients treated in the hospital’s dermatology department and found that none was infected with COVID-19. This observation prompted researchers to evaluate hydroxychloroquine in vitro for the treatment and prevention of COVID-19. Not only did hydroxychloroquine treat cells infected with the virus that causes COVID-19, but the drug also protected cells from becoming infected.8
This research provides basic science evidence that hydroxychloroquine should be considered for both the prevention and treatment of COVID-19.
Clinical research. Clinical research suggests that hydroxychloroquine alone and potentially in conjunction with the antibiotic azithromycin can be used to treat patients with COVID-19.
A report published online ahead of print in February indicated that the early clinical experience with both hydroxychloroquine and chloroquine for patients with COVID-19 was very positive.9 In a more recent randomized study from China that evaluated 62 hospitalized patients with COVID-19, 80.6% (25 of 31) treated with hydroxychloroquine 200 mg twice per day for 5 days experienced an improvement in their pneumonia (as evaluated by computed tomography scan) versus 54% (17 of 31) of COVID-19 patients on placebo.7 Fever was also eliminated more rapidly (2.2 vs 3.2 days) in treated versus untreated patients, respectively.
Based on the in vitro activity of hydroxychloroquine on SARS-CoV-2, Didier Raoult, MD, PhD, of Marseille Medical School in France, initiated two clinical trials. The first, an open-label nonrandomized study, enrolled 36 hospitalized patients with confirmed COVID-19.10 Of the 20 who were treated with 600 mg hydroxychloroquine for 10 days, six also received azithromycin 500 mg on the first day, followed by 250 mg daily for 4 additional days. On day 6 (Figure 2), more polymerase chain reaction swabs in nasopharyngeal samples from patients on chloroquine (57.1%) or chloroquine plus azithromycin (100%) tested negative compared to those obtained from the control group (12.5%).
Figure 2. Results from the open-label nonrandomized clinical trial by Dr. Raoult, which found that hydroxychloroquine with or without azithromycin had a higher percentage of virologic cure at day 6, as determined by a negative polymerase chain reaction.
In the second study by Dr. Raoult, 80 consecutive patients were treated with a combination of oral hydroxychloroquine 600 mg administered daily for 10 days and azithromycin 500 mg administered on the first day, followed by 250 mg daily for 4 additional days.11 Because the combination of these two drugs can induce an arrhythmia, patients were given a baseline electrocardiogram, followed by a second one on day 2 of treatment. No patient developed a cardiac arrythmia. Of the patients, 81.3% were discharged from the hospital (mean time, 5 days). The health of two of the three patients transferred to the ICU improved, and they were subsequently able to leave the ICU. One patient died before being transferred to the ICU. Overall, the nasopharyngeal swabs obtained from 93% of patients on day 10 of treatment tested negative.
LONG HALF-LIFE, BUT POTENTIAL DOWNSIDE
There is now a strong interest in the use of hydroxychloroquine for both treatment and prevention of COVID-19. One unique characteristic of hydroxychloroquine is its long half-life (40 days). In other words, after taking a prescribed amount of the drug, it takes more than 6 weeks for the level of hydroxychloroquine found in the body to drop by 50%. Therefore, individuals who are placed on hydroxychloroquine will have it in their system for a prolonged time.
The main downside of hydroxychloroquine therapy is the uncommon but serious risk of a cardiac arrythmia due to QT prolongation. A risk-scoring system that has been developed can help physicians determine a patient’s risk of drug-induced QT prolongation.12
HOW IT WORKS
With a growing body of evidence supporting hydroxycholroquine’s clinical effectiveness against COVID-19, it is helpful to explore how it actually works. Hydroxychloroquine is believed to have a dual mechanism of action against COVID-19 and potentially a third immunomodulating effect.
No. 1: Hydroxychloroquine raises the pH level of endosomes, membrane-bound structures within cells through which viruses can enter the human body. Raising the pH likely disrupts the ability of COVID-19 to enter human cells. No. 2: Hydroxychloroquine increases the intracellular concentration of zinc.13-15 Zinc blocks the viral replication of coronavirus, so the combination of hydroxychloroquine and zinc can help stop the virus from growing within cells. No. 3: Hydroxychloroquine is a known immunomodulator. As the infection from COVID-19 progresses and becomes more severe, one of the deadliest possible consequences is a cytokine storm that induces severe inflammation. Cytokines IL-1, IL-6, and Interferon gamma have all been implicated in multisystem organ failure, including cardiac and renal failure and coagulopathy, in critically ill COVID-19 patients. Data in the literature suggest that hydroxychloroquine may help to regulate these cytokines and prevent the cytokine storm.
 Understanding the basic science on how hydroxychloroquine works in COVID-19 provides compelling support on why this medication should be considered for both prophylaxis and treatment.
DRUG AVAILABILITY
Although many countries have been waiting for clinical trial results, in January, Malaysia started treating COVID-19 patients routinely with hydroxychloroquine.16 Italy has now also begun providing hydroxychloroquine to all patients who are diagnosed with COVID-19.17 Multiple clinical trials are ongoing in the United States, including an observational study that is attempting to enroll more than 1,000 patients in New York. In the meantime, the FDA has issued an Emergency Use Authorization for hydroxychloroquine, which allows physicians to prescribe hydroxychloroquine to hospitalized patients.18
A number of pharmaceutical companies have increased production of hydroxychloroquine. On March 29, Sandoz, a division of Novartis, provided 30 million tablets to the US Department of Health and Human Services.19 Plenty of raw materials are also available to continue manufacturing hydroxychloroquine for the US population.
ON THE FRONT LINES
First responders and health care workers such as nurses, technicians, and physicians who are caring for patients who may have COVID-19 should speak with their own doctors and consider the risks and benefits of prophylactic hydroxychloroquine therapy.
Because individuals infected with COVID-19 can remain asymptomatic for 5 to 10 days, first responders and health care workers may not realize they are infected and can thus unknowingly transmit COVID-19 to their patients, coworkers, and family. It is important to know that infected individuals can experience atypical symptoms that include conjunctivitis, diarrhea, heart pain (angina), and a loss of taste or smell (anosmia). Health care workers who know that they have been exposed to a patient with COVID-19 but who have not tested positive for the virus can consider joining an ongoing randomized clinical trial of hydroxychloroquine prophylaxis sponsored by the University of Minnesota.
CONCLUSION
A growing body of evidence suggests that hydroxychloroquine can be used for both prophylaxis and treatment in individuals infected with COVID-19. The optimal dose of the drug is unknown, but a review of the basic science and clinical research indicates that a dose of 400 mg daily for 5 to 6 days, followed by 400 mg once per week can serve as prophylaxis. For patients with a confirmed COVID-19 infection, the French protocol of oral hydroxychloroquine 600 mg daily for 10 days plus azithromycin 500 mg on the first day, followed by 250 mg daily for 4 additional days, can be considered. Since this combination increases the risk of an arrhythmia, consultation with one’s physician before initiating this regimen is important. Other therapies such as vitamin C, vitamin B3, and zinc may also play a role in prophylaxis and treatment.
Rather than wait for symptoms to develop, it may be beneficial to achieve a therapeutic level of hydroxychloroquine in one’s system if there is an increased risk for exposure, such as in first responders, health care workers, or someone who came into contact with a COVID-19–positive individual. The hope is that the hydroxychloroquine onboard would minimize the severity of the COVID-19 infection and reduce the chance of requiring hospitalization.
Because treatment with hydroxychloroquine has been shown in clinical studies to rapidly shorten the period in which patients test positive for COVID-19,7 therapy may also shorten the number of days that these patients can transmit the infection to others. If so, this would be of critical benefit to first responders and health care workers who are at high risk of exposure and whose rapid recovery can allow them to resume serving the public during this crisis. Risk of toxicity, such as retinal toxicity, is relatively low with short-term use of hydroxychloroquine. Nevertheless, the higher initial COVID-19 dosing of the drug and the evolving paradigms of treatment duration—particularly if used multiple times for postexposure prophylaxis—may warrant caution and necessitate preemptive evaluation with a baseline retinal function testing and follow-on monitoring.
----------------------
Understanding the basic science on how hydroxychloroquine works in COVID-19 provides compelling support on why this medication should be considered for both prophylaxis and treatment.
DRUG AVAILABILITY
Although many countries have been waiting for clinical trial results, in January, Malaysia started treating COVID-19 patients routinely with hydroxychloroquine.16 Italy has now also begun providing hydroxychloroquine to all patients who are diagnosed with COVID-19.17 Multiple clinical trials are ongoing in the United States, including an observational study that is attempting to enroll more than 1,000 patients in New York. In the meantime, the FDA has issued an Emergency Use Authorization for hydroxychloroquine, which allows physicians to prescribe hydroxychloroquine to hospitalized patients.18
A number of pharmaceutical companies have increased production of hydroxychloroquine. On March 29, Sandoz, a division of Novartis, provided 30 million tablets to the US Department of Health and Human Services.19 Plenty of raw materials are also available to continue manufacturing hydroxychloroquine for the US population.
ON THE FRONT LINES
First responders and health care workers such as nurses, technicians, and physicians who are caring for patients who may have COVID-19 should speak with their own doctors and consider the risks and benefits of prophylactic hydroxychloroquine therapy.
Because individuals infected with COVID-19 can remain asymptomatic for 5 to 10 days, first responders and health care workers may not realize they are infected and can thus unknowingly transmit COVID-19 to their patients, coworkers, and family. It is important to know that infected individuals can experience atypical symptoms that include conjunctivitis, diarrhea, heart pain (angina), and a loss of taste or smell (anosmia). Health care workers who know that they have been exposed to a patient with COVID-19 but who have not tested positive for the virus can consider joining an ongoing randomized clinical trial of hydroxychloroquine prophylaxis sponsored by the University of Minnesota.
CONCLUSION
A growing body of evidence suggests that hydroxychloroquine can be used for both prophylaxis and treatment in individuals infected with COVID-19. The optimal dose of the drug is unknown, but a review of the basic science and clinical research indicates that a dose of 400 mg daily for 5 to 6 days, followed by 400 mg once per week can serve as prophylaxis. For patients with a confirmed COVID-19 infection, the French protocol of oral hydroxychloroquine 600 mg daily for 10 days plus azithromycin 500 mg on the first day, followed by 250 mg daily for 4 additional days, can be considered. Since this combination increases the risk of an arrhythmia, consultation with one’s physician before initiating this regimen is important. Other therapies such as vitamin C, vitamin B3, and zinc may also play a role in prophylaxis and treatment.
Rather than wait for symptoms to develop, it may be beneficial to achieve a therapeutic level of hydroxychloroquine in one’s system if there is an increased risk for exposure, such as in first responders, health care workers, or someone who came into contact with a COVID-19–positive individual. The hope is that the hydroxychloroquine onboard would minimize the severity of the COVID-19 infection and reduce the chance of requiring hospitalization.
Because treatment with hydroxychloroquine has been shown in clinical studies to rapidly shorten the period in which patients test positive for COVID-19,7 therapy may also shorten the number of days that these patients can transmit the infection to others. If so, this would be of critical benefit to first responders and health care workers who are at high risk of exposure and whose rapid recovery can allow them to resume serving the public during this crisis. Risk of toxicity, such as retinal toxicity, is relatively low with short-term use of hydroxychloroquine. Nevertheless, the higher initial COVID-19 dosing of the drug and the evolving paradigms of treatment duration—particularly if used multiple times for postexposure prophylaxis—may warrant caution and necessitate preemptive evaluation with a baseline retinal function testing and follow-on monitoring.
----------------------
- Dong Y, Mo X, Hu Y, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. March 2020. doi: 10.1542/peds.2020-0702.
- Survey shows just how dire PPE shortages are at many hospitals. Medscape. https://www.medscape.com/viewarticle/927728. Accessed April 1, 2020.
- Wang D, Hu B, Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069.
- https://bloomberg.com/news/articles/2020-03-17/Europe-s-doctors-getting-sick-like-in-wuhan-chinese-doctors-say. Bloomberg News. Accessed April 2, 2020.
- Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69.
- Biot C, Daher W, Chavain N, et al. Design and synthesis of hydroxyferroquine derivatives with antimalarial and antiviral activities. J Med Chem. 2006;49(9):2845-2849.
- Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRxiv 2020. doi: 10.1101/2020.03.22.20040758.
- Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 9 March 2020. doi: 10.1093/cid/ciaa237.
- Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. BioScience Trends. 2020;14(1):72-73.
- Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. In Press. 17 March 2020. doi: 10.1016/j.ijantimicag.2020.105949.
- Gautret P, Lagier JC, Rarola P, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: an observational study. https://www.mediterranee-infection.com/wp-content/uploads/2020/03/COVID-IHU-2-1.pdf. Accessed April 2, 2020.
- Simpson T. Ventricular arrhythmia risk due to hydroxychloroquine-azithromycin treatment for COVID-19. Cardiology Magazine. https://www.acc.org/latest-in-cardiology/articles/2020/03/27/14/00/ventricular-arrhythmia-risk-due-to-hydroxychloroquine-azithromycin-treatment-for-covid-19. Accessed April 1, 2020.
- Aartjan J, Velthuis SH, et al. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLOS Pathog. 2010;6(11): e1001176.
- Read SS, Obeid S, Ahlenstiel C, Ahlenstiel G. The role of zinc in antiviral immunity. Advances in Nutrition. 2019;10(4):696-710.
- Xue J, Moyer A, Peng B, Wu J, Hannafon BN, Ding WQ. Chloroquine is a zinc ionophore. PLoS One. 2014;9(10):e109180.
- Chloroquine’ used to treat Covid-19 patients since first wave, says Health D-G. Malay Mail Newspaper. https://www.malaymail.com/news/malaysia/2020/03/29/chloroquine-used-to-treat-covid-19-patients-since-first-wave-says-health-d/1851457. Accessed April 1, 2020.
- Italy finally starts mass treatment with hydroxychloroquine. March 29, 2020. Trust Nodes website. https://www.trustnodes.com/2020/03/29/italy-finally-starts-mass-treatment-with-hydroxychloroquine. Accessed April 1, 2020.
- Request for emergency use authorization for use of chloroquine phosphate or hydroxychloroquine sulfate supplied from the strategic national stockpile for treatment of 2019 coronavirus disease. Letter from US Food and Drug Administration. https://www.fda.gov/media/136534/download. Accessed March 30, 2020.
- HHS accepts donations of medicine to strategic national stockpile as possible treatments for COVID-19 patients. Letter from US Department of Health and Human Services. https://www.hhs.gov/about/news/2020/03/29/hhs-accepts-donations-of-medicine-to-strategic-national-stockpile-as-possible-treatments-for-covid-19-patients.html. Accessed April 1, 2020.
Here the last mortality data from “Istituto Superiore della Sanità” (technical-scientific body of the National Health Service in Italy): it carries out research, experimentation and training on health issues.
Statistics based on 16.000 cases.
https://www.epicentro.iss.it/en/coronavirus/bollettino/Report-COVID-2019_9_april_2020.pdf
Well rounded article. Mature comments.
Link: https://blogs.sciencemag.org/pipeline/archives/2020/05/15/good-news-on-the-human-immune-response-to-the-coronavirus